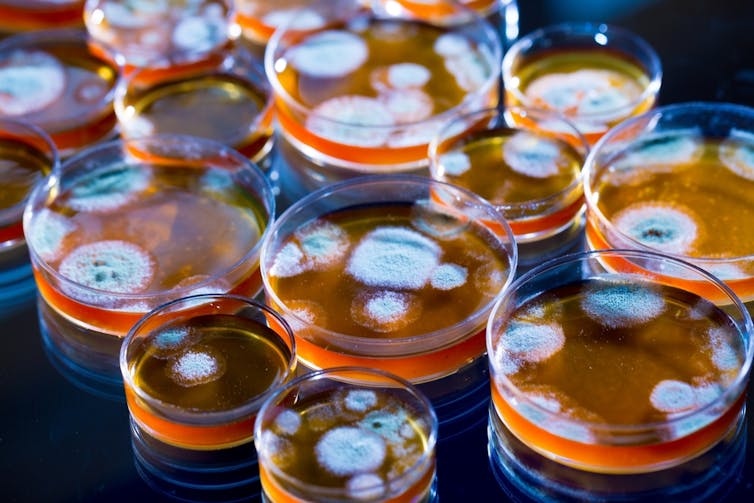
In 1928 Alexander Fleming discovered penicillin, thus bringing one of the greatest medical advances of our time: antibiotics.
Innocuous infections, operations and injuries were no longer a death sentence.
Since then, antibiotics have been developed to treat an array of diseases but this slowed, and then stopped in the 1980s. Although our arsenal of development ceased, the bacteria, viruses and fungi did not stop evolving.
This asymmetric development has resulted in an antimicrobial resistance problem: bacteria causing common infections and illnesses are now increasingly resistant to the drugs used to treat them.

Creative Commons Attribution 4.0 International Public License. Attribution notice: ‘Review on Antimicrobial Resistance.’ From the O’Neill Report in 2016.
Solving this issue is not straightforward.
It involves a complex landscape of policy makers, clinicians, vets, law makers, and many others.
As part of Bristol Doctoral College’s Research without Borders Festival 2017, a public discussion was held exploring the problem of superbugs and antibiotic resistance, in both the context of research happening at the university of Bristol, and from a wider perspective.
Discussions revolved around patent law, and how it may affect development of new drugs and solutions, the role of agriculture, in particular dairy farming, in reducing antimicrobial resistance, and what we can do as individuals to help address this problem.
Below is a brief snapshot of the research relating to antimicrobial resistance being undertaken across the University of Bristol by the postgraduate researchers who took part in the RWB discussion panel. Continue reading →