In 1928 Alexander Fleming discovered penicillin, thus bringing one of the greatest medical advances of our time: antibiotics.
Innocuous infections, operations and injuries were no longer a death sentence.
Since then, antibiotics have been developed to treat an array of diseases but this slowed, and then stopped in the 1980s. Although our arsenal of development ceased, the bacteria, viruses and fungi did not stop evolving.
This asymmetric development has resulted in an antimicrobial resistance problem: bacteria causing common infections and illnesses are now increasingly resistant to the drugs used to treat them.

Creative Commons Attribution 4.0 International Public License. Attribution notice: ‘Review on Antimicrobial Resistance.’ From the O’Neill Report in 2016.
Solving this issue is not straightforward.
It involves a complex landscape of policy makers, clinicians, vets, law makers, and many others.
As part of Bristol Doctoral College’s Research without Borders Festival 2017, a public discussion was held exploring the problem of superbugs and antibiotic resistance, in both the context of research happening at the university of Bristol, and from a wider perspective.
Discussions revolved around patent law, and how it may affect development of new drugs and solutions, the role of agriculture, in particular dairy farming, in reducing antimicrobial resistance, and what we can do as individuals to help address this problem.
Below is a brief snapshot of the research relating to antimicrobial resistance being undertaken across the University of Bristol by the postgraduate researchers who took part in the RWB discussion panel.
Antibiotics & Biology
Juan Carlos Jimenez Castellanos
Currently, bacteria which can resist the effect of multiple antibiotics (a phenomenon known as multidrug resistance, or antimicrobial resistance) is an increasingly life threatening issue.
There are different reasons why bacteria can become resistant to these drugs, for example, the misuse of antibiotics within livestock or within hospitals exerts an intense selective pressure upon bacteria that leads to the development of resistance. Likewise, different molecular and biological pathways are involved in the development of resistance.
As an example, it has been described that protein in the bacterial cell walls, known as efflux pumps, are able to extrude different compounds, including antibiotics, from within the bacteria cell. As a result, the antibiotic(s) do not have the desired effect against bacteria (i.e. killing it).
Knowing the biology and the physiology of the bacterial cell has allowed us to understand bacterial behaviour.
This has led to the development of new antimicrobial molecules which can work in combination with existing antibiotics, as a new line of therapy for bacterial infections. So far, we have been able to characterise different bacterial traits not only at molecular level but also some of the roles that the environment plays.
The phenomenon of resistance is very dynamic and it spans disciplines beyond the biological ones.
Having a general knowledge of how antimicrobial resistance evolves, constructed from different perspectives, will help to tackle multidrug resistant bacteria, and this global sharing of knowledge base is a key aspect in the fight, recommended in the O’Neill Report in 2016.
Antibiotics in Healthcare
Sarah Garner
In recent years, more and more people have become aware of the problems of overuse of antibiotics in healthcare.
Once seen as a ‘cure all’ for many conditions, their use is now becoming increasingly restricted, in an effort to try and stop the development of antimicrobial resistance: a situation where ‘the drugs don’t work’.
Headlines screaming of MRSA in hospital wards, and patients with common infections spending weeks in intensive care, serve as reminders of what could happen in the future if we don’t address this issue.
A world where common infections are life threatening, where even the simplest operation carries a risk of dying, where a cancer diagnosis is, once again, a death sentence, either from the surgery or the immune system weakening chemotherapy.
The problem in a nutshell, is that bacteria evolve much quicker than we do, or our ability to design new antibiotics does. No new antibiotics have come to the market since the 1980s, whilst a new infectious disease has been discovered nearly every year since then.
Developing innovative approaches to tackling the problem of antibiotic resistance is key, and it has become apparent in recent years that a more ‘joined up’ approach is required.
Multidisciplinary input is needed, from understanding the molecular mechanisms by which bacteria develop resistance, to collecting data on how antibiotics are used in healthcare and agriculture globally, to re-examining law and national policies, which can influence the direction research and innovation take.
One such approach is the development or redesign of existing antiseptics (rather than antibiotics), which already have a good track record in healthcare, and to which bacteria cannot develop resistance.
Another is to use physical means – rather than the traditional chemical or biological mechanisms of antibiotics – to kill bacteria: taking inspiration from the shape of surfaces in nature, which kill bacteria by physically piercing or causing them to rupture, such as cicada wings, or shark skin.
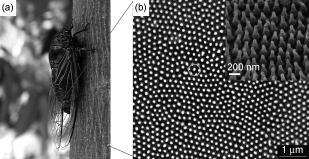
Cicada Wing Surface showing antibacterial nanopillar structures. From: Zhang et al. Cicada Wings: A Stamp from Nature for Nanoimprint Lithography. Small (2006) 2: 12; 1440–1443.
Reducing the use of antibiotics: saving them for the really important situations, alongside these innovations, may just give us a chance to face the next 30 years with some ways to circumnavigate the problem of antimicrobial resistance, and to be able to continue to use antibiotics when we really need them.
Again, this was highlighted in the O’Neill Report, but to what degree this report can influence policy both in the UK and internationally, remains to be seen.
Antibiotics and agriculture
Gwen Rees
Mention the words ‘antibiotics’ and ‘agriculture’ in the same sentence, and many people take a sharp intake of breath and shake their heads.
Few realise that the majority of antibiotics in the UK are actually used in human medicine (~55%).
That being said, antibiotics are used in food producing animals for the treatment or prevention of disease, and it is vitally important that they are used responsibly.
Many steps have been taken in the last 20 years to bring about positive change in this area, however the O’Neill Report emphasised the need for improved surveillance and measurement of antibiotic use and called for a global target for the reduction of antibiotic use in agriculture.
The government’s response to the report promised sector-specific targets for antimicrobial use but did not address the issue of a lack of granularity and detail from surveillance data in the animal health sector.
There are several initiatives currently working towards this goal from stakeholders including Responsible Use of Medicines in Agriculture Alliance (RUMA), Cattle Health and Welfare Group (CHAWG) and the University of Bristol’s AMR Force, amongst others, looking at different ways of gathering and analysing this data, and policy-makers should look to these working groups for future policy development.
In the UK at the moment, the amount of antibiotics used is only really known at the national level, with the pig and poultry industry using the most veterinary antibiotics by a significant amount.
Beef and dairy cattle come in third on the list, but there is very little data available at the farm or animal level about the quantity of antibiotics and which type are actually going into these animals.

Picture of collection bins for used antibiotic containers from dairy farms as part of her research into antibiotics and agriculture, courtesy of Gwen Rees.
Without this level of detail, it is very difficult to design and implement effective policy to promote the responsible use of antibiotics in agriculture.
By introducing policy that specifically requires veterinary practice-level or even farm-level prescription data, and supporting the implementation, it would enable benchmarking of antibiotic use and targeted reduction of certain critically important antimicrobials in agriculture.
The aim of the UK agricultural sector has always been to produce high quality food from happy, healthy animals.
With an improved understanding of the way antibiotics are used, combined with engaged and motivated farmers, future policy changes should be able to achieve this goal in combination with responsible use of antibiotics and a reduced risk of transmission of antibiotic resistance.
Antibiotics and Patent Law
Louise Hatherall
In 2013 Dame Sally Davis, the Chief Medical Officer of the UK argued that global action is required to tackle antibiotic resistance.
Part of this global challenge was to encourage more innovation for the development of new antibiotics to tackle increasing resistance by disease.
One way to encourage innovation is through patents.
Patents are monopolies granted over inventions, for a set period, to exclude all other users from utilising the claimed invention. This monopoly can then allow the inventor to exploit the invention for profit, or simply to carry on researching without fear of competition.
Patents can cover a wide range of inventions: from the lab equipment used to research novel ways of producing new antibiotics, to designs stemming from synthesised molecules.
Patents are complex legal objects, claimed to both encourage and stifle innovation.
Without patents, companies may not have the financial incentive to develop new antibiotics. However, their power to exclude others from using their protected inventions may result in researchers being unable to access and research the latest developments.
This could arguably stagnate research.
Patents over antibiotics and medicines have also been linked to increased prices for drugs resulting in drugs which are potentially too expensive for clinician and patient access.
There is a burgeoning field of research in this area as lawyers, scientists and other interested parties work toward a solution.
However, science and law are both complicated, technical areas which often struggle to relay information between one another.
My research seeks to understand how these two areas communicate with one another, and whether patent law is helping or hindering the development and dissemination of ways to tackle antibiotic resistance.
Authors: Juan Carlos Jimenez Castellanos, Doctor of Philosophy Student, School of Cellular and Molecular Medicine; Sarah Garner, Academic Clinical Fellow, School of Oral and Dental Sciences; Louise Hatherall, Doctor of Philosophy Student, University of Bristol Law School; and Gwen Rees, Doctor of Philosophy Student, School of Veterinary Sciences.
